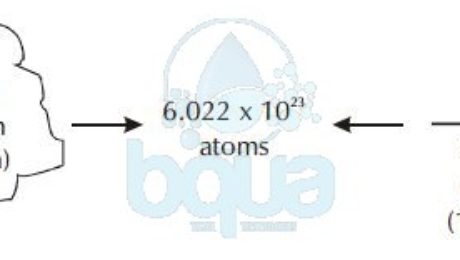
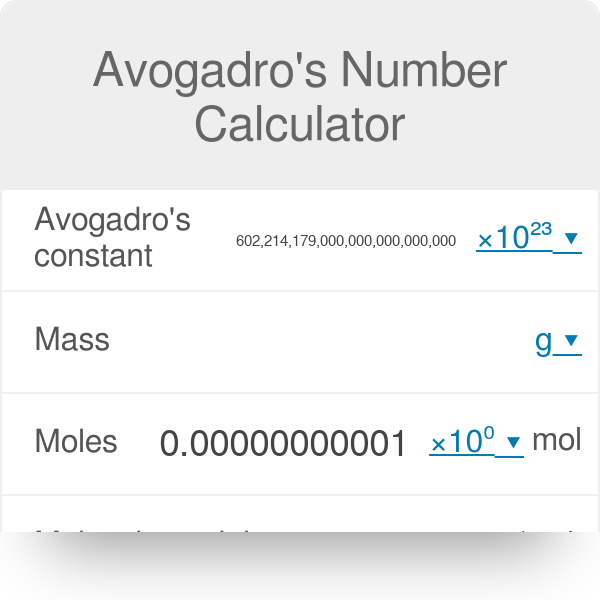
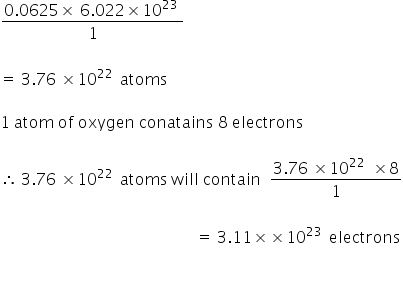12 g C - 12 contains 6.022 × 10^23 atoms of carbon.(a) 6.022 × 10^23 is known as .............(b) Calculate the number of carbon atoms present in 48 g C - 12.(c)

If Avogadro number NA is changed from 6.022 × 10^23 mol^-1 to 6.022 × 10^20 mol^-1 , this would change :

Calculate the mass of 6 022 x 10^23 molecules of CaCO3 - Science - Atoms and Molecules - 13283691 | Meritnation.com

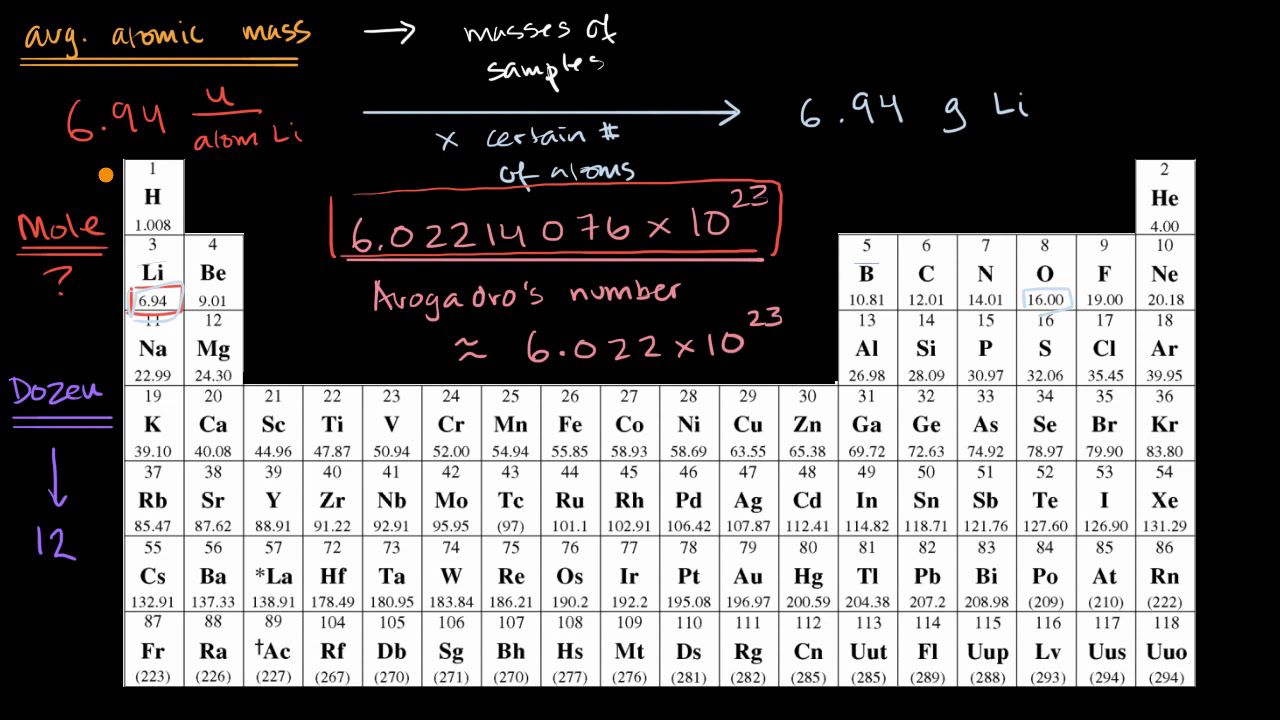
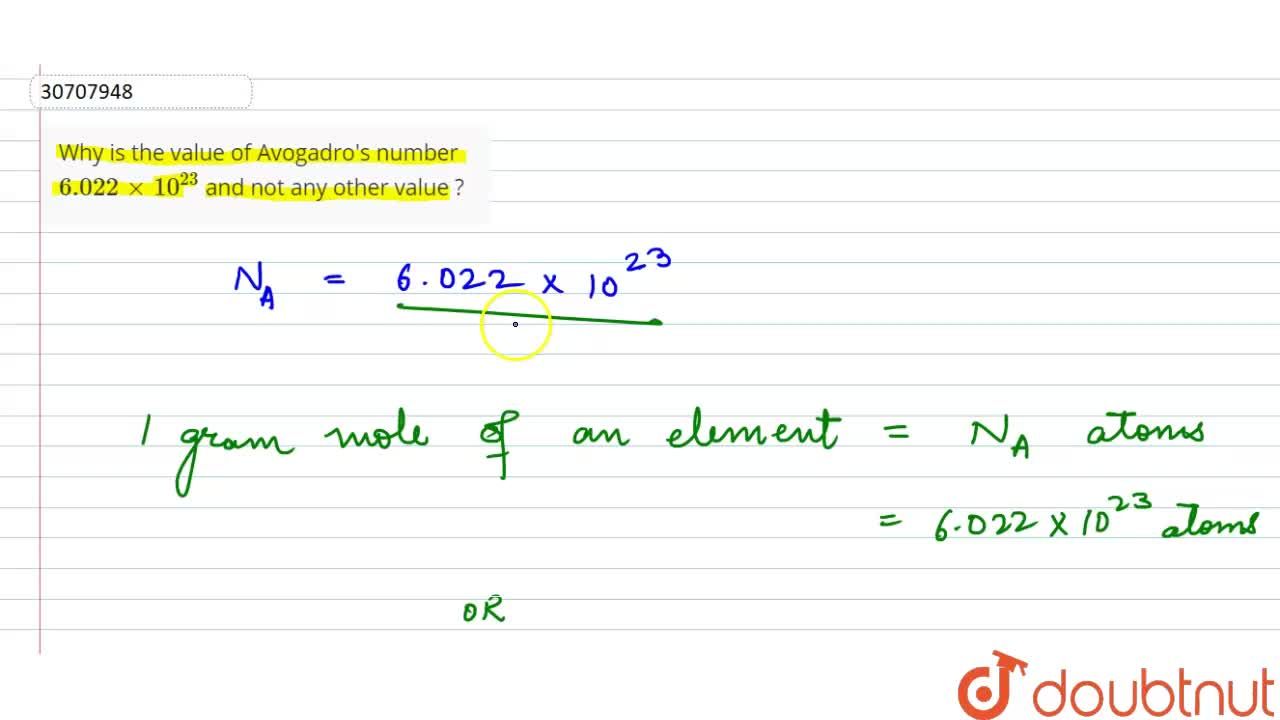
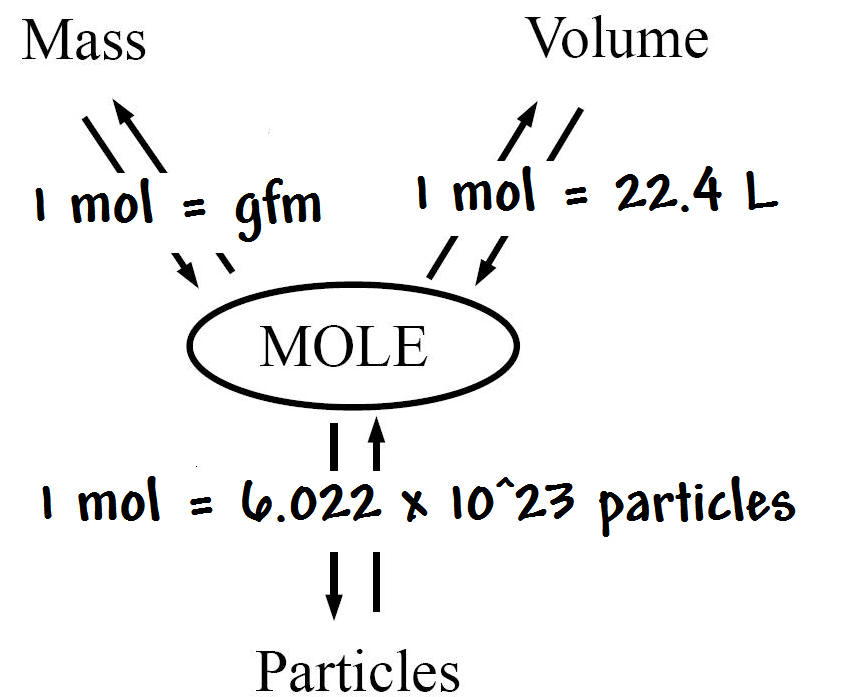
A mole is a collection of 6.022 xx 10^23 particles and the number 6.022 xx 10^23 is called Avogadro number. The mass of this number of atoms in an element is equal

Oh My!!. Mole (mol) can be defined as the number equal to the number of carbon atoms in grams of carbon (in an chemical equation it is the coefficients. - ppt download

Atomic Mass and The Mole Topic: AMU's & Atomic Mass Objectives: Day 1 of 3 To learn how we define 1 amu (atomic mass unit) To learn how we derive atomic. - ppt download
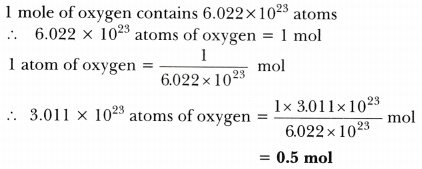
Calculate the number of moles present in: 3.011 X 10^23 number of oxygen atoms. 60 g of calcium - CBSE Class 9 Science - Learn CBSE Forum

One mole of any substance contains 6.022xx10^(23) atoms/molecules. Number of molecules of H(2)SO(4) present in 100mL of 0.02M H2SO(4) solution is

Which of the following contains the same number of atoms as 4.032g of hydrogen atoms? A. 1 mole of H2 - Brainly.com
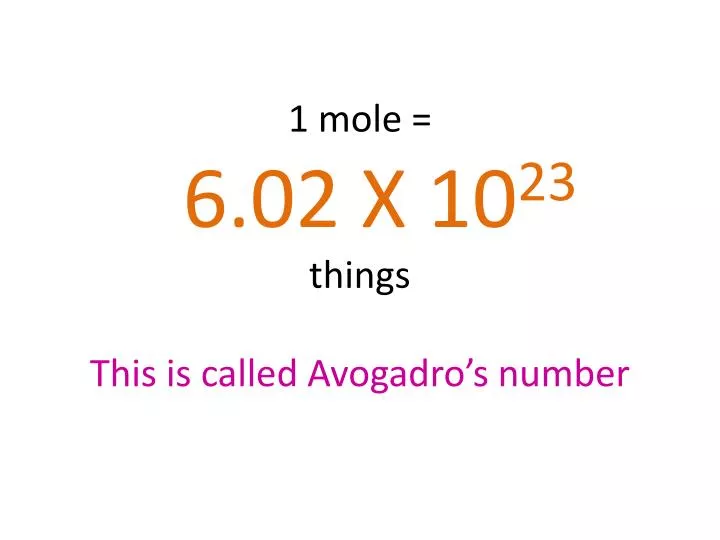
PPT - 1 mole = 6.02 X 10 23 things This is called Avogadro's number PowerPoint Presentation - ID:4272623